Scientific Documentation
A Randomized, Double-Blind, Placebo- Controlled Study Examining the Effects of a Rapidly Soluble Chitosan Dietary Supplement on Weight Loss and Body Composition in Overweight and Mildly Obese Individuals
- Rebecca N. Schiller, MS, CN, Eleanor Barrager, RD,
- Alexander G. Schauss, PhD, Everett J. Nichols, MSPH, PhD
Download the full study here
Goal
The goal of this study is to evaluate the efficacy of a rapidly-soluble chitosan (LipoSan Ultra™) in facilitating weight loss and reducing body fat in overweight and mildly obese individuals consuming a high-fat diet. This study also examines functional changes in gastrointestinal and elimination symptoms caused by supplementation of this compound.
Methods
Fifty nine (59) overweight, mildly obese, otherwise healthy females with a history of daily dietary fat consumption greater than or equal to 30% of total calories participated in a randomized double-blind placebo-controlled trial. Subjects received either three (3) capsules of a rapidly soluble chitosan (LipoSan Ultra™) or matched placebo, twice daily for eight (8) weeks.
Supplementation was divided into two doses, 1.5 g each, and was taken just prior to the two largest meals of the day. No food restrictions or modifications were assigned, and subjects were instructed to continue their regular caloric intake. Dietary calorie and fat intake was monitored during the study by three-day diet diaries, completed at baseline and at Weeks 4 and 8. Anthropometric measurements and functional gastrointestinal and elimination symptoms were measured at baseline and Week 8.
Results
Mean weight, body mass index (BMI), and percent body fat increased significantly in the placebo group as compared to baseline (p<0.01, 0.001, and 0.05 respectively); additionally, percent lean body mass decreased significantly within the placebo group (p<0.005). Within the treatment group, both mean weight and BMI decreased significantly (p<0.005 and 0.05 respectively) compared to baseline. The mean weight loss within the treatment group was 1.0 kg over an eight-week period.
This was in contrast to the placebo group, which experienced a mean weight gain of 1.5 kg over the same time period. When comparing study groups, endpoint values for mean weight and BMI were significantly higher in the placebo group (p<0.0001 and 0.01 respectively). Chi-square analysis for weight loss between groups indicates significantly more subjects lost weight within the treatment group than within the placebo group (63% and 17% respectively, p<0.001). No significant changes in functional gastrointestinal and elimination symptoms occurred in either group.

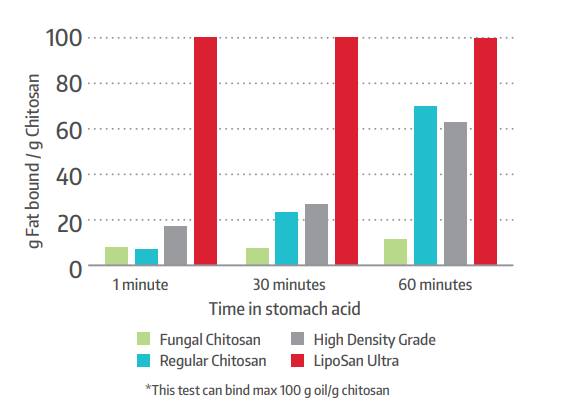
Conclusion
Our findings suggest that this rapidly-soluble chitosan (LipoSan Ultra™) is efficacious in facilitating weight loss and reducing body fat in overweight and mildly obese individuals. In addition, we found no significant changes in gastrointestinal and elimination symptoms with the use of this polysaccharide biopolymer.
Primex has access to a vast collection of studies and tests that we are happy to share with potential customers. Please contact us for further information.